ISSUE1634
- Mark Abramowicz, M.D., President: no disclosure or potential conflict of interest to report
- Jean-Marie Pflomm, Pharm.D., Editor in Chief: no disclosure or potential conflict of interest to report
- Brinda M. Shah, Pharm.D., Consulting Editor: no disclosure or potential conflict of interest to report
- Review the influenza vaccines available in the US for the 2021-2022 season and discuss which vaccines are appropriate for specific patient populations.
- Discuss the risks and benefits associated with annual vaccination against influenza.
- Annual vaccination against influenza A and B viruses is recommended for everyone ≥6 months old without a contraindication.
- Vaccination should be offered by the end of October and continue to be offered as long as influenza viruses are circulating in the community.
- All influenza vaccines available in the US this season are quadrivalent; they contain two influenza A and two influenza B virus antigens.
- Influenza vaccination reduces the risk of influenza illness and can lower the risk of influenza-related hospitalizations and death.
- Use of a recombinant, high-dose, or adjuvanted vaccine can improve immune responses in older adults.
- Pregnant women should be vaccinated against influenza without regard to the trimester of pregnancy.
- Persons with a history of egg allergy can receive any age-appropriate influenza vaccine.
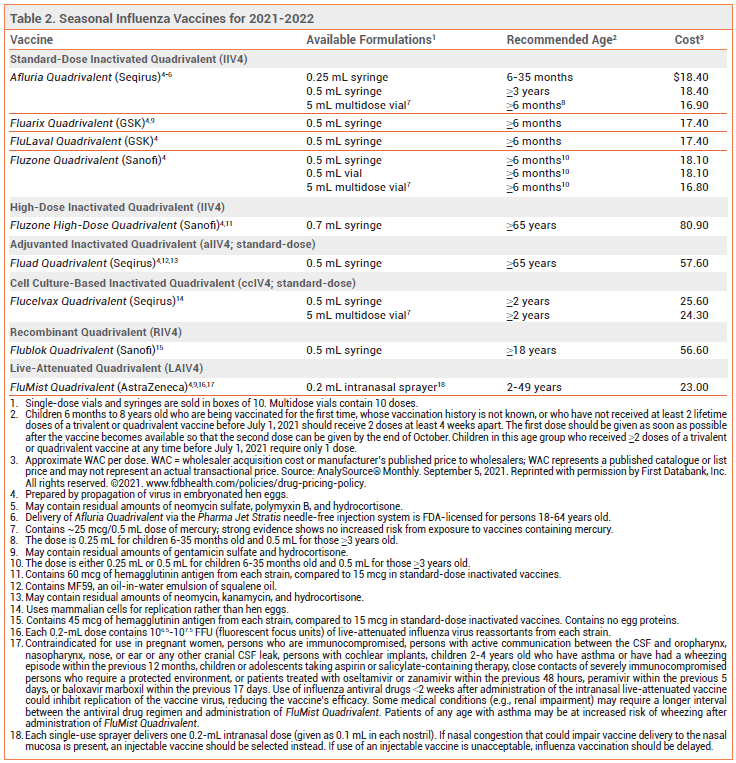
Annual vaccination against influenza A and B viruses is recommended for everyone ≥6 months old without a contraindication.1 Available influenza vaccines for the 2021-2022 season are listed in Table 2.
Vaccination of all eligible persons can reduce the prevalence of influenza illness and symptoms that might be confused with those of COVID-19. Lower-than-usual rates of influenza illness have been observed this season in the Southern Hemisphere, probably because of masking, social distancing, school closures, and travel restrictions.2 According to the CDC, reduced immunity due to low influenza virus activity since March 2020 may result in an early and possibly severe influenza season in 2021-2022.1
TIMING — In the US, vaccination against influenza should be offered by the end of October and continue to be offered as long as influenza is circulating in the community. In most adults, serum antibody levels peak about two weeks after vaccination. Early vaccination (i.e., in July or August) may result in suboptimal immunity before the end of the influenza season, especially in older adults.3 Children who require 2 doses (see Table 2, footnote 2) should receive the first dose as early as possible so that the second dose can be given by the end of October. Vaccination of persons with suspected or laboratory-confirmed COVID-19 should be postponed until they are no longer acutely ill and no longer require isolation.
COMPOSITION — Influenza A viruses are the main cause of influenza-related morbidity and mortality, particularly in infants and older adults. Children are more likely than adults to become infected with influenza B.
All influenza vaccines available in the US this season are quadrivalent; they contain two influenza A and two influenza B virus antigens (see Table 1).
EFFECTIVENESS — Influenza vaccination reduces the incidence of laboratory-confirmed influenza and can reduce the risk of serious complications and death associated with influenza illness in children and adults.4-7 The effectiveness of the seasonal influenza vaccine in preventing influenza illness depends on several factors, including the match between the vaccine and circulating strains and the immunologic response of the recipient. Vaccine effectiveness is greatest when the match is close, but even when it is suboptimal, vaccination can still substantially reduce the risk of influenza-related hospitalization and death.8,9
LIVE-ATTENUATED VACCINE — FluMist Quadrivalent, the intranasal live-attenuated influenza vaccine, is FDA-approved for use in healthy nonpregnant persons 2-49 years old (see Table 2, footnote 17 for contraindications).
OLDER ADULTS — Older adults may have weaker immunogenic responses to influenza vaccination than younger persons, and their antibody levels may decline more rapidly, decreasing vaccine effectiveness.10 In a recent trial in community-dwelling adults 65-82 years old, recombinant, high-dose, and adjuvanted vaccines improved humoral and cell-mediated immune responses compared to standard-dose inactivated vaccines.11 Clinical trials comparing the three types of vaccines in older adults are lacking.
Recombinant Vaccine – Flublok Quadrivalent, a recombinant influenza vaccine produced without the use of influenza virus or chicken eggs, contains 3 times the amount of antigen included in standard-dose inactivated influenza vaccines.
In a randomized, double-blind trial in 8604 adults ≥50 years old during the A/H3N2-predominant 2014-2015 season, the recombinant quadrivalent vaccine was 30% more effective than a nonadjuvanted standard-dose inactivated quadrivalent vaccine in preventing laboratory-confirmed influenza illness.12
High-Dose Vaccine – Fluzone High-Dose Quadrivalent, an inactivated vaccine that contains 4 times the amount of antigen included in standard-dose inactivated influenza vaccines, is FDA-licensed for use in persons ≥65 years old.
In a randomized, double-blind trial in 31,989 adults ≥65 years old during 2 influenza seasons, a high-dose inactivated trivalent vaccine (Fluzone High-Dose – not available in 2021-2022) induced significantly greater antibody responses than a standard-dose inactivated trivalent vaccine and was 24% more effective in preventing laboratory-confirmed influenza illness.13 In several studies in adults ≥65 years old, a high-dose inactivated trivalent vaccine was associated with a reduced risk of respiratory-related and all-cause hospitalization and death compared to standard-dose inactivated trivalent vaccines.14-17
In a randomized trial in 5260 patients with high-risk cardiovascular disease, use of a high-dose inactivated trivalent vaccine over 3 influenza seasons did not significantly reduce all-cause mortality or cardiopulmonary hospitalizations compared to standard-dose inactivated quadrivalent vaccines.18
Adjuvanted Vaccine – The adjuvanted inactivated influenza vaccine Fluad Quadrivalent is FDA-licensed for use in persons ≥65 years old. It contains MF59, an oil-in-water emulsion of squalene oil that increases the immune response by recruiting antigen-presenting cells to the injection site and promoting uptake of influenza virus antigens.
In a randomized trial in 7082 adults ≥65 years old, an adjuvanted inactivated trivalent vaccine (Fluad – not available in 2021-2022) elicited significantly greater antibody responses against all three influenza strains than a nonadjuvanted inactivated trivalent vaccine.19 In several trials, older adults who received an adjuvanted inactivated trivalent vaccine were less likely than those who received a nonadjuvanted inactivated trivalent vaccine to develop symptomatic influenza illness or to be hospitalized for influenza or pneumonia.20-22
PREGNANCY — The ACIP and the American College of Obstetricians and Gynecologists (ACOG) recommend that pregnant women be vaccinated against influenza without regard to the trimester of pregnancy.23,24 Vaccination protects pregnant women against influenza-associated illness, which can be especially severe during pregnancy, and protects their infants for up to the first 6 months after birth (influenza vaccines are not approved for use in infants <6 months old).25
Most studies have not found an association between influenza vaccination and adverse pregnancy outcomes, but data demonstrating the safety of vaccination during the first trimester are limited.26 Pregnant women should not receive the live-attenuated vaccine.
EGG ALLERGY — Although most influenza vaccines contain trace amounts of egg protein (ovalbumin), numerous studies have demonstrated that patients with egg allergy are not at increased risk for a reaction to any influenza vaccine.27 The recombinant vaccine (Flublok Quadrivalent) and the cell culture-based vaccine (Flucelvax Quadrivalent) do not contain egg protein.
The ACIP states that persons with egg allergy of any severity can receive any age-appropriate influenza vaccine, but those with a history of more severe egg allergy (angioedema, respiratory distress, light-headedness, recurrent vomiting, or requiring epinephrine or another emergency medical intervention) who receive an egg-based vaccine should be vaccinated in a medical setting (e.g., doctor's office or clinic) supervised by a clinician experienced in managing severe allergic reactions.1
The Joint Task Force on Practice Parameters of the American Academy of Allergy Asthma and Immunology and the American College of Allergy Asthma and Immunology as well as the American Academy of Pediatrics state that no special precautions are necessary for patients with egg allergy of any severity.28
IMMUNOCOMPROMISED PERSONS — The live-attenuated influenza vaccine should not be used in immunocompromised persons. Inactivated and recombinant vaccines are generally considered safe for use in such persons, but the immune response may be reduced. In two randomized trials in solid-organ transplant recipients, the high-dose vaccine induced significantly greater immune responses than standard-dose vaccines.29,30 Separation in time of influenza vaccination from an immunocompromising intervention might be considered.
USE WITH OTHER VACCINES — Any influenza vaccine can be given at the same time as a COVID-19 vaccine, but the vaccines should be administered at different sites. Inactivated and recombinant influenza vaccines can be administered concomitantly or sequentially with live or other inactivated or recombinant vaccines. The live-attenuated influenza vaccine can be given simultaneously with inactivated or other live vaccines; other live vaccines not administered simultaneously should be given at least 4 weeks later. Use of a nonadjuvanted influenza vaccine could be considered in persons receiving an adjuvanted non-influenza vaccine (e.g., Shingrix, Heplisav-B); coadministration of Shingrix and a nonadjuvanted inactivated quadrivalent influenza vaccine has not been associated with a decrease in the immunogenicity of either vaccine.31
USE WITH INFLUENZA ANTIVIRALS — Use of oseltamivir (Tamiflu, and generics) or zanamivir (Relenza) within 48 hours before, peramivir (Rapivab) within 5 days before, or baloxavir marboxil (Xofluza) within 17 days before or <2 weeks after administration of the intranasal live-attenuated influenza vaccine could inhibit replication of the vaccine virus, reducing the vaccine's efficacy.
ADVERSE EFFECTS — Influenza vaccination has been associated with Guillain-Barré syndrome, but the absolute risk is very low (about 1-2 additional cases per million persons vaccinated), and influenza infection itself has been associated with the syndrome (about 17 cases per million influenza infection encounters).32,33
Except for soreness at the injection site, adverse reactions to inactivated influenza vaccines are uncommon. In clinical trials, Fluzone High-Dose (trivalent formulation) caused more injection-site reactions than standard-dose influenza vaccines. Pain and tenderness at the injection site also occurred more frequently with Fluad (adjuvanted trivalent) than with a nonadjuvanted vaccine. Delivery of Afluria by needle-free jet injector has resulted in more mild to moderate local reactions than delivery of a vaccine by standard needle and syringe.
The most common adverse reactions associated with the live-attenuated vaccine are runny nose, nasal congestion, fever, and sore throat. The vaccine can increase the risk of wheezing, especially in children <5 years old with recurrent wheezing and in persons of any age with asthma. Persons who receive the live-attenuated vaccine may shed the vaccine-strain virus for a few days after vaccination, but person-to-person transmission has been rare, and serious illness resulting from transmission has not been reported. Nevertheless, persons who care for severely immunocompromised patients in protected environments should not receive the live-attenuated vaccine or should avoid contact with such patients for 7 days after receiving it.
- LA Grohskopf et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 Influenza Season. MMWR Recomm Rep 2021; 70(No. RR-5):1. Available at: https://bit.ly/3BPK9dS. Accessed September 9, 2021.
- World Health Organization. Influenza update N° 399. August 2, 2021. Available at: https://bit.ly/3l4TXcY. Accessed September 9, 2021.
- JM Ferdinands et al. Waning vaccine effectiveness against influenza-associated hospitalizations among adults, 2015- 2016 to 2018-2019, United States hospitalized adult influenza vaccine effectiveness network. Clin Infect Dis 2021; 73:726.
- C Arriola et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis 2017; 65:1289.
- B Flannery et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics 2017; 139:e20164244.
- AP Campbell et al. Effect of vaccination on preventing influenza-associated hospitalizations among children during a severe season associated with B/Victoria viruses, 2019-2020. Clin Infect Dis 2021; 73:e947.
- S Garg et al. Influenza vaccine reduces risk of severe outcomes among adults hospitalized with influenza A(H1N1)pdm09, FluServ-NET, 2013-2018. ID Week, Washington, DC, October 2-6, 2019. Abstract 898. Available at: https://bit.ly/3lIEr6e. Accessed September 9, 2021.
- M Darvishian et al. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis 2014; 14:1228.
- MA Rolfes et al. Effects of influenza vaccination in the United States during the 2017-2018 influenza season. Clin Infect Dis 2019; 69:1845.
- B Young et al. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 2017; 35:212.
- BJ Cowling et al. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a randomized, controlled trial. Clin Infect Dis 2020; 71:1704.
- LM Dunkle et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376:2427.
- CA DiazGranados et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635.
- S Gravenstein et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 2017; 5:738.
- DK Shay et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012-2013 and 2013-2014. J Infect Dis 2017; 215:510.
- JKH Lee et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines 2018; 17:435.
- Y Young-Xu et al. High-dose influenza vaccination and mortality among predominantly male, white, senior veterans, United States, 2012/13 to 2014/2015. Euro Surveill 2020; 25:1900401.
- O Vardeny et al. Effect of high-dose trivalent vs standard-dose quadrivalent influenza vaccine on mortality or cardiopulmonary hospitalization in patients with high-risk cardiovascular disease: a randomized clinical trial. JAMA 2021; 325:39.
- SE Frey et al. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014; 32:5027.
- PG Van Buynder et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013; 31:6122.
- F Lapi et al. Adjuvanted versus nonadjuvanted influenza vaccines and risk of hospitalizations for pneumonia and cerebro/cardiovascular events in the elderly. Expert Rev Vaccines 2019; 18:663.
- KW McConeghy et al. Cluster-randomized trial of adjuvanted vs. non-adjuvanted trivalent influenza vaccine in 823 U.S. nursing homes. Clin Infect Dis 2020 September 4 (epub).
- CDC. Addressing concerns pregnant people might have about influenza vaccine safety. September 3, 2021. Available at: https://bit.ly/3jDkTyg. Accessed September 9, 2021.
- ACOG Committee Opinion No. 732: influenza vaccination during pregnancy. Obstet Gynecol 2018; 131:e109.
- MG Thompson et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010-2016. Clin Infect Dis 2019; 68:1444.
- A Mehrabadi et al. Association of maternal influenza vaccination during pregnancy with early childhood health outcomes. JAMA 2021; 325:2285.
- M Greenhawt et al. Administration of influenza vaccines to egg allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol 2018; 120:49.
- American Academy of Allergy Asthma & Immunology. Egg allergy and the flu vaccine. August 11, 2021. Available at: https://bit.ly/3yV2uUI. Accessed September 9, 2021.
- Y Natori et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66:1698.
- AG L'Huillier et al. Cell-mediated immune responses after influenza vaccination of solid organ transplant recipients: secondary outcomes analyses of a randomized controlled trial. J Infect Dis 2020; 221:53.
- TF Schwarz et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis 2017; 216:1352.
- JC Kwong et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13:769.
- LL Polakowski et al. Chart-confirmed Guillain-Barré syndrome after 2009 H1N1 influenza vaccination among the Medicare population, 2009-2010. Am J Epidemiol 2013; 178:962.