ISSUE1727
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
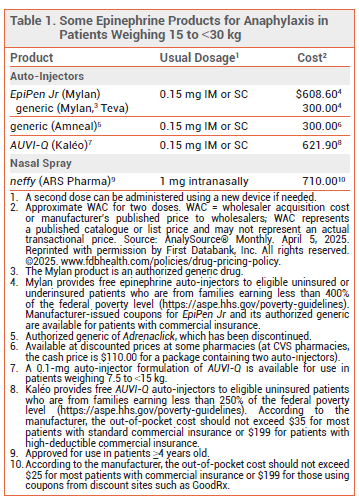
The FDA has approved a 1-mg epinephrine nasal spray (neffy - ARS Pharma) for emergency treatment of type 1 hypersensitivity reactions including anaphylaxis in patients ≥4 years old who weigh 15 to <30 kg. A 2-mg formulation of neffy was approved in 2024 for use in patients who weigh ≥30 kg.1
CLINICAL STUDIES — FDA approval of the 1-mg formulation of neffy was based on the results of a pharmacologic study (summarized in the package insert) that included 21 children ≥4 years old who weighed 15 to <30 kg. Geometric mean plasma epinephrine concentrations in the children after a 1-mg dose were higher than those in a reference group of adults after a 2-mg dose.
ADVERSE EFFECTS — Adverse effects of the 1-mg dose included nasal and upper respiratory tract congestion, dry throat, nasal dryness, and paresthesia. The nasal spray solution contains sodium metabisulfite, which could cause a hypersensitivity reaction in patients with a sulfite allergy, but a history of sulfite sensitivity should not deter emergency use of the product.
DOSAGE, ADMINISTRATION, AND COST — The recommended dosage of neffy in children ≥4 years old who weigh 15 to <30 kg is one 1-mg spray administered into one nostril. If symptoms have not improved after 5 minutes, a second spray can be administered into the same nostril using a new device. The nasal spray should be stored at room temperature, but excursions up to 50° C (122° F) are permitted. At temperatures below -15° C (5° F), the solution freezes and the device cannot deliver epinephrine. The shelf life of neffy 1 mg is 24 months (the 2-mg formulation has a shelf life of 30 months).
The 1-mg formulation of neffy is expected to be available in May 2025. According to the manufacturer, the out-of-pocket cost for two neffy nasal spray devices should not exceed $25 for most patients with commercial insurance.2
- An epinephrine nasal spray (neffy) for anaphylaxis. Med Lett Drugs Ther 2024; 66:163.
- ARS Pharma Press Release. ARS Pharmaceuticals announces FDA approval of neffy 1 mg (epinephrine nasal spray) for type I allergic reactions, including anaphylaxis, in pediatric patients weighing 15 to <30 kilograms. March 5, 2025. Available at: https://bit.ly/4kLwQ5a. Accessed April 10, 2025.
